
A staff member checks the packaging quality of COVID-19 inactivated vaccine products at a packaging plant of the Beijing Biological Products Institute Co., Ltd. in Beijing, capital of China, Dec. 25, 2020. (Xinhua/Zhang Yuwei)
BEIJING, Jan.1 (Xinhua)-- On the last day of 2020, China said that it had granted conditional marketing authorization for its first self-developed COVID-19 vaccine, which will be provided free of charge to all Chinese people.
This is also great news for the world that has been shadowed by the COVID-19 pandemic throughout 2020.
The following is a timeline of the key moments from China's endeavors to develop COVID-19 vaccines.
April 14
China has approved three COVID-19 vaccine candidates for clinical trials, according to an official with the Ministry of Science and Technology.
An adenovirus vector vaccine, developed by a research team led by Chen Wei, an academician with the Chinese Academy of Engineering and a researcher at the Institute of Military Medicine under the Academy of Military Sciences, was the first to be approved to enter clinical trials, said the official.

President Xi Jinping learns about the progress on the vaccine and anti-body during his visit to the Academy of Military Medical Sciences in Beijing, capital of China, March 2, 2020. (Xinhua/Ding Haitao)
May 22
China's vaccine trial has been found to be safe, well-tolerated, and able to generate an immune response against SARS-COV-2 in humans, according to a study published online by medical journal The Lancet.
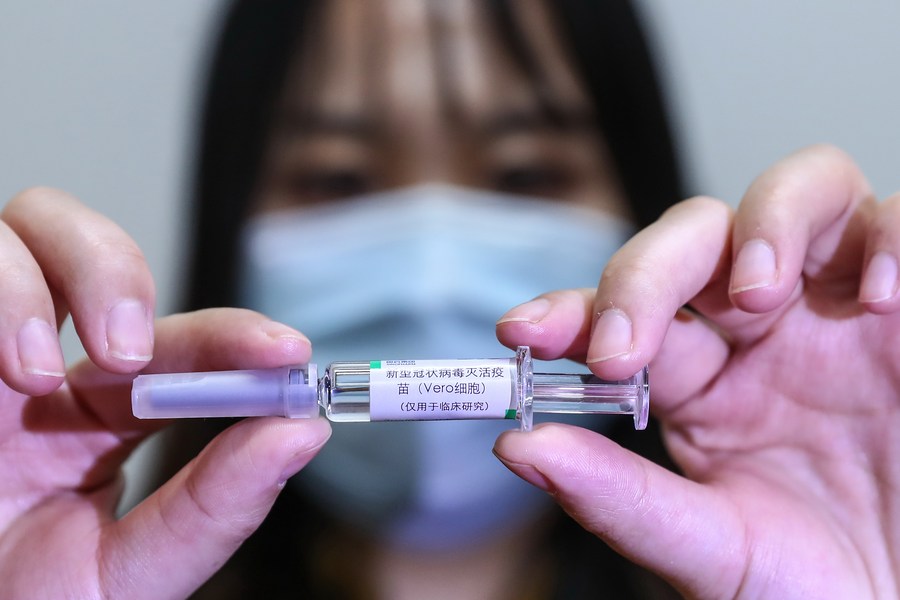
A staff member displays a sample of the COVID-19 inactivated vaccine at a vaccine production plant of China National Pharmaceutical Group Co., Ltd. (Sinopharm) in Beijing, capital of China, April 10, 2020. (Xinhua/Zhang Yuwei)
Jun. 24
China's inactivated COVID-19 vaccine candidate has started its phase-3 clinical trial globally in the United Arab Emirates, according to the vaccine developer China National Biotec Group (CNBG).
It was the first time that China's self-developed vaccine has carried out phase-3 clinical research internationally.
July 22
To protect high-risk groups, China's central government authorized emergency use of the COVID-19 vaccines.
Aug. 17
China's first patent for a COVID-19 vaccine has been granted by the National Intellectual Property Administration.

An exhibitor shows a 3D model of inactivated COVID-19 vaccine antigen to visitors at the booth of SINOVAC in the special area for public health and epidemic prevention in the comprehensive exhibition area of the 2020 China International Fair for Trade in Services (CIFTIS) in Beijing, capital of China, Sept. 5, 2020. (Xinhua/Zhang Yuwei)
Oct.8
China signed an agreement with Gavi, the Vaccine Alliance, officially joining COVAX. This is an important step China has taken to uphold the concept of a shared community of health for all and to honor its commitment of turning COVID-19 vaccines into a global public good.
Nov. 18
China has five vaccines for COVID-19 that are currently undergoing phase III clinical trials in the United Arab Emirates, Brazil, Pakistan and Peru, and some other vaccines that are undergoing phase I and II clinical trials, according to Foreign Ministry spokesperson Zhao Lijian.
Dec. 2
China had five COVID-19 vaccines entering phase-3 clinical trials by Dec.2.
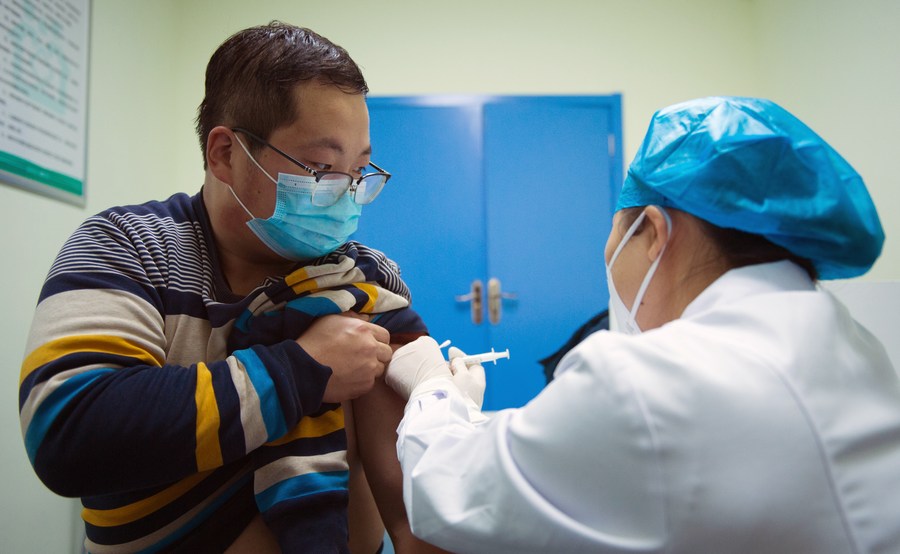
A staff member of an airport receives a shot of the COVID-19 vaccine at Dizhang health center, in Xixian New Area, northwest China's Shaanxi Province, Dec. 25, 2020. Shaanxi started inoculating some key groups with COVID-19 vaccines on Friday. (Xinhua/Wei Xiang)
Dec.20
More than one million doses of COVID-19 vaccines have been distributed for emergency inoculation use since July, said Zheng Zhongwei, an official with the National Health Center.
Dec. 24
The city of Wuhan in central China started the emergency use of COVID-19 vaccine candidates. According to the center for disease control and prevention in Wuhan, vaccinations are available at 48 designated clinics in 15 districts, targeting key groups of people aged between 18 and 59.
Dec. 25,
Northwest China's Shaanxi Province started administering COVID-19 vaccines. Groups being inoculated include those involved in customs inspections and quarantine, port border inspection and cold chain logistics, as well as medical staff.
Dec. 26
The Shanghai Municipal Center for Disease Control and Prevention said the city has started COVID-19 inoculation among key groups of front-line workers, while vaccinations for people traveling abroad for work or study purposes will begin in due course.
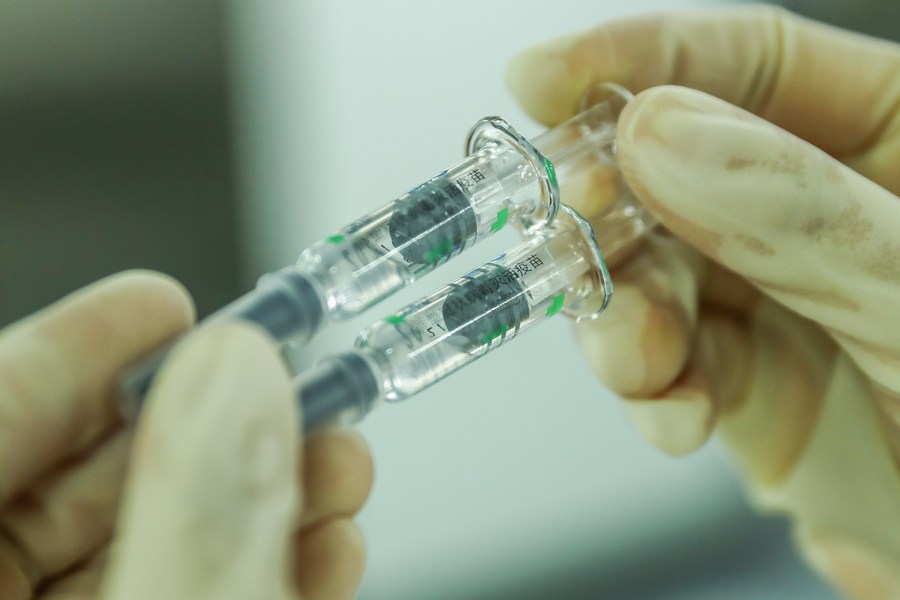
A staff member checks the packaging quality of COVID-19 inactivated vaccine products at a packaging plant of the Beijing Biological Products Institute Co., Ltd. in Beijing, capital of China, Dec. 25, 2020. China announced on Thursday that it had granted conditional marketing authorization for its first self-developed COVID-19 vaccine. (Xinhua/Zhang Yuwei)
Dec.31
China approves first self-developed COVID-19 vaccine
- It will be provided free of charge to all Chinese people
- It shows 79.34 percent efficacy
- China will vaccinate senior citizens, people with underlying conditions & general public in an orderly manner. ■




